NHS Virtual Wards: How AI Triage Supports Remote Monitoring
Discover how AI-powered triage systems are transforming NHS Virtual Wards, enabling effective remote patient monitoring, reducing hospital readmissions, and improving healthcare outcomes across the UK.


The National Health Service (NHS) is at a critical juncture, facing unprecedented demand, capacity constraints, and the imperative to modernise its care delivery models. In response, the NHS Virtual Ward initiative has emerged as a cornerstone of national strategy, aiming to provide hospital-level acute care to patients in their own homes. This model, underpinned by a significant £450 million initial investment and a national ambition to establish up to 24,000 virtual beds, represents a paradigm shift from traditional, building-centric healthcare to a more flexible, patient-centred, and digitally enabled system.
This report provides a comprehensive strategic analysis of the integration of Artificial Intelligence (AI) within the NHS Virtual Ward framework, focusing on its role in enhancing remote patient monitoring (RPM) through intelligent triage and risk stratification. Our analysis reveals that AI is not merely an add-on but a critical enabler that performs two distinct but interconnected functions: first, a population-level triage through predictive risk stratification to identify and proactively admit the most vulnerable patients onto the virtual ward; and second, a continuous patient-level triage of incoming RPM data to detect early signs of deterioration and direct clinical attention where it is most needed.
The technological core of the virtual ward is an RPM ecosystem of patient-facing apps, medical devices, and clinician dashboards. AI transforms this ecosystem from a passive data collection system into a proactive clinical management tool. By leveraging machine learning algorithms, these systems can analyse vast, continuous streams of physiological data to predict adverse events, filter out non-critical alerts to reduce clinician cognitive load, and automate routine patient engagement. Evidence from NHS Trusts demonstrates the tangible benefits of this approach, with case studies reporting significant reductions in hospital admissions and readmissions—by as much as 45% in some pilots—alongside high levels of patient satisfaction.
However, the rapid, policy-driven rollout of this model is running ahead of a robust, national-level evidence base. The economic case remains contested, with conflicting reports on cost-effectiveness, a debate hampered by a lack of standardised metrics and the inherent difficulty of comparing the cost of a unit of workforce capacity (a "virtual bed") with the shared overhead of a physical hospital bed.
Furthermore, significant implementation challenges threaten to undermine the initiative's potential. These are not primarily technological but are deeply rooted in human and systemic factors. A critical finding of this report is that digital equity is not a peripheral concern but a core determinant of the model's viability; a failure to address barriers related to digital literacy, connectivity, and social support will exclude a significant portion of the target patient population, thereby capping the model's impact on system capacity. Simultaneously, the opaque "black box" nature of some AI algorithms creates a fundamental tension with the principle of clinical accountability, forming a major barrier to trust and adoption among frontline staff. Overcoming this requires a strategic focus on explainable AI (XAI) and the development of a clear national framework for liability.
To realise the transformative potential of AI-enabled virtual wards, this report puts forward a series of strategic recommendations. Policymakers must prioritise investment in foundational digital infrastructure over standalone technologies and establish clear regulatory guidance on AI accountability. NHS Leaders must champion co-design implementation models with staff and patients, invest in new workforce skills, and demand greater transparency from technology vendors. Finally, Technology Developers must focus on seamless interoperability and the validation of algorithms on diverse UK datasets to mitigate bias.
The AI-supported virtual ward is more than a technological innovation; it is a critical stepping stone towards a future healthcare paradigm defined by proactive, predictive, and personalised care. Successfully navigating the complex challenges outlined in this report will be essential for the NHS to secure a sustainable and effective future.
The NHS Virtual Ward Initiative: A New Paradigm for Acute Care
The concept of the virtual ward, or "Hospital at Home," has rapidly evolved from a niche innovation to a central pillar of the NHS's strategy for managing acute care demand. Driven by systemic pressures and a growing recognition of the benefits of home-based recovery, this model represents a fundamental rethinking of the hospital's role and physical boundaries. This section defines the virtual ward, details its operational mechanics, and outlines the governance and patient cohorts it is designed to serve, establishing the foundational context for the technological and AI-driven layers that enable its function.
1.1 From Hospital Bed to Home: Defining the Virtual Ward
At its core, a virtual ward provides hospital-level care to patients in their usual place of residence, which can be their own home or a care home. NHS England formally defines it as "a safe and efficient alternative to bedded hospital care that is enabled by technology". This model is designed for patients who are acutely unwell and would otherwise require admission to a physical hospital bed. The care is delivered by a dedicated, multidisciplinary team (MDT) who provide a range of tests and treatments, including blood tests, intravenous therapies, and daily clinical reviews, mirroring the standard of care provided in a traditional hospital setting.
The strategic impetus behind the national rollout is a direct response to the immense and growing pressures on NHS capacity. The primary goals are to prevent avoidable hospital admissions, support earlier and safer discharge, reduce the overall length of patient stays in hospital, and ultimately free up physical beds for those with the most critical needs. This objective is explicitly stated as a key ambition in the NHS's 'Delivery plan for recovering urgent and emergency care services'.
Beyond systemic efficiency, the model is built on a strong patient-centric philosophy. A significant body of evidence suggests that patients often prefer to recover in the familiar and comfortable environment of their own home. This preference is linked to tangible clinical and wellbeing benefits; being at home facilitates contact with family, friends, and pets, which is shown to be beneficial for mental health and overall recovery rates. Furthermore, it helps patients avoid the known risks of prolonged hospitalisation, such as hospital-acquired infections, deconditioning, falls, and delirium. Indeed, evidence suggests patients are eight times less likely to experience functional decline in a virtual ward compared to an equivalent inpatient stay. This positive experience is reflected in patient satisfaction surveys, with over 99% of patients on existing virtual wards stating they would recommend the service.
The scale of this strategic commitment is substantial. NHS England has set a national ambition to create 40–50 virtual ward "beds" per 100,000 population, which would equate to a total national capacity of approximately 24,000 beds. This rollout is supported by an initial investment of £450 million over two years. As of December 2023, every integrated care system (ICS) in England had introduced virtual wards, reaching a capacity of around 11,800 beds. On an average day, approximately 8,600 of these beds were occupied, representing a national occupancy rate of just under 73%.
A crucial nuance in understanding this scale is the definition of a "virtual bed." Unlike a physical bed in a hospital, a virtual bed is not a piece of furniture but an abstract measure of the clinical workforce's capacity to safely manage one patient remotely. This distinction is fundamental. It reframes the challenge of scaling virtual wards from a problem of capital investment in technology to one of strategic workforce planning, recruitment, and training for a highly skilled, mobile clinical team. This conceptual difference also complicates direct cost comparisons with traditional inpatient care, as a "virtual bed" represents a fully-costed, dedicated unit of clinical service, whereas an inpatient bed cost is often calculated as a share of a hospital's vast and fixed overheads.
1.2 Operational Blueprint: 'Step-Up' and 'Step-Down' Models
The operational framework for virtual wards is built around two primary patient pathways, designed to integrate seamlessly with existing urgent and emergency care services: the 'step-up' and 'step-down' models.
'Step-Up' (Admission Avoidance): This model functions as a direct alternative to hospital admission. Patients are referred—or 'stepped up'—to the virtual ward from various points in the community, including their GP, Urgent Community Response (UCR) teams, Same Day Emergency Care (SDEC) units, or directly from an Emergency Department (ED) assessment. The objective is to provide the necessary acute care at home, thereby preventing an inpatient admission altogether.
'Step-Down' (Early Supported Discharge): This model is designed to reduce the length of stay for patients already in hospital. A patient who is clinically stable but not yet medically optimised for a full discharge can be transferred—or 'stepped down'—from a physical hospital ward to a virtual ward. This allows them to return home sooner while continuing to receive the required level of acute monitoring, treatment, and specialist oversight until their episode of care is complete.
The application of these models is not uniform across all clinical conditions, reflecting a necessary tailoring of the service to meet specific patient needs. This has led to a diverse landscape of virtual ward implementations across the country, with significant variation in referral pathways, staffing models, and the use of technology. For example, an analysis of NHS data from April 2023 revealed that frailty virtual wards are predominantly 'step-up' services, with 76% of their patients being admitted from the community to avoid hospitalisation. In contrast, wards for acute respiratory infections (ARI) and circulatory diseases show a more balanced profile, with a greater proportion of patients being 'stepped down' from hospital to facilitate early discharge. This operational diversity highlights a key strategic tension: while NHS England has established a standardised national framework and targets, the reality of successful implementation is highly localised and context-dependent. This makes national-level evaluation complex and suggests that a rigid, one-size-fits-all approach to scaling may be less effective than a flexible framework that empowers local systems to adapt the model to their unique demographic and clinical needs.
1.3 The Patient Profile: Governance and Eligibility
Admission to a virtual ward is governed by strict clinical criteria to ensure patient safety and appropriateness of care. The fundamental principle is that a patient must be "sick enough to otherwise be in hospital". This distinguishes virtual wards from other community health services; the acuity of the patients is high enough to warrant consultant-level oversight.
Each virtual ward operates under the leadership of a named and accountable consultant physician or practitioner, who may be a doctor, specialist nurse, or Allied Health Professional (AHP) with consultant-level practice. The patient is cared for by a full MDT, which can include senior nurses, social workers, pharmacists, and community nurses, who conduct daily ward rounds (either virtually or in-person) and have access to hospital-level diagnostics and interventions. The expected length of stay on a virtual ward is short-term, typically between one and 14 days, after which the patient is discharged back to the care of their GP.
The model has been established for a range of acute conditions, with specific pathways developed for key patient cohorts.
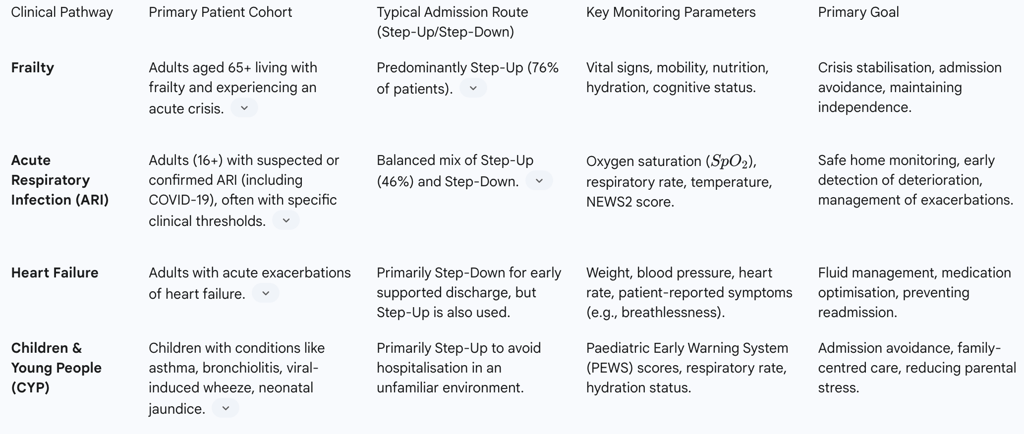

Table 1: Comparison of NHS Virtual Ward Models
In all cases, a critical and overarching inclusion criterion is the informed choice of the patient or their carer. The patient must understand the model of care and agree that they wish to receive their treatment at home rather than in a hospital. This principle of shared decision-making is central to the virtual ward's ethos, positioning it not just as a tool for system efficiency but as a means of delivering more personalised and patient-led care.
The Technological Core: Enabling At-Home Care Through Remote Patient Monitoring (RPM)
The operational viability of the NHS Virtual Ward initiative is entirely dependent on technology. Remote Patient Monitoring (RPM) forms the digital backbone of the service, creating a continuous link between the patient at home and the clinical team. This technological ecosystem allows for the safe management of acutely unwell patients outside the hospital walls by facilitating data collection, clinical oversight, and timely intervention. This section deconstructs the components of the RPM system and examines the clinical workflows it enables.
2.1 The Digital Umbilical Cord: The RPM Ecosystem
A technology-enabled virtual ward platform is not a single piece of software but an integrated ecosystem comprising three essential components: patient-facing technology, a suite of medical devices, and a central digital platform for healthcare professionals.
Patient-Side Technology: Upon admission to a virtual ward, the patient (or their carer) is provided with a kit of straightforward medical devices tailored to their specific clinical needs. This typically includes a pulse oximeter (to measure blood oxygen levels and heart rate), a blood pressure monitor, and a digital thermometer. For certain conditions, such as heart failure, digital weighing scales may also be included to monitor for fluid retention. The patient is asked to take a set of observations at clinically defined intervals, usually several times a day.
These readings are then transmitted to the clinical team via a patient-facing application on a smartphone or a dedicated tablet. Technology providers such as Masimo (SafetyNet app) and Inhealthcare offer platforms that guide the patient through the process of taking and submitting their readings. To ensure digital inclusivity, these platforms often support multiple communication channels, allowing patients to submit data not just through an app but also via simple SMS text messages or automated telephone calls, where they can key in their results.
Clinician-Side Technology: The patient-submitted data is fed in real-time into a secure digital platform or dashboard, accessible to the virtual ward's MDT. This platform provides a comprehensive, at-a-glance view of the entire patient caseload. A key feature of these dashboards, offered by providers like Graphnet and Inhealthcare, is the use of a RAG (Red, Amber, Green) status for each patient. This colour-coding system automatically flags patients whose readings are outside of pre-set parameters or who are showing worrying trends, allowing clinicians to instantly identify those who require the most urgent attention. The platform also enables clinicians to view historical data and trends, providing a richer and more dynamic picture of a patient's condition than the intermittent spot-checks possible on a busy hospital ward.
2.2 The Flow of Information: Clinical Workflows and Communication
The RPM ecosystem enables a new and highly efficient clinical workflow that blends remote oversight with traditional hands-on care. The cornerstone of this workflow is the daily "virtual ward round". Instead of a physical procession around a hospital ward, the clinical team reviews each patient's data on the digital dashboard. This is typically followed by direct contact with the patient via a telephone or video call to discuss their progress, symptoms, and the monitoring results.
The system's primary function is to facilitate proactive, rather than reactive, care. The technology is designed to provide an early warning of clinical deterioration. When a patient's submitted observations breach the defined clinical thresholds, or if a negative trend is identified over several readings, the system generates an automated alert that is immediately flagged to the clinical team. This allows the team to intervene swiftly, for example by providing advice over the phone, arranging for an urgent home visit, or, if necessary, organising readmission to hospital. This continuous stream of objective data can enable clinicians to identify a deteriorating patient long before the patient themselves might feel unwell enough to seek help, a capability that has been credited with saving lives.
It is crucial to recognise that this model is not entirely remote. It is a blended approach that combines high-tech monitoring with high-touch, in-person care. The technology acts as a sophisticated information filter, enabling a limited clinical workforce to manage a geographically dispersed group of patients efficiently. The insights generated by the RPM data guide the deployment of the MDT, ensuring that face-to-face visits are directed to the patients who need them most. This "wraparound care" model ensures that while the patient benefits from the comfort of home, they are never disconnected from the expert oversight of a hospital-level clinical team.
The success of this entire technological framework, however, is contingent on the "human infrastructure" that supports it. The devices and dashboards are merely enablers; their value is only realised through the 24/7 availability of a skilled clinical team ready to interpret the data, respond to alerts, and provide both virtual and physical care. The powerful case study of the COVID-19 virtual wards demonstrated that the technology's primary role was to allow clinicians to "identify and focus on the 8.4% of patients who needed to be readmitted," thereby directing scarce human expertise precisely where it was most needed. Therefore, any strategy to scale up virtual ward capacity must involve a parallel scaling of the associated clinical response teams.
Furthermore, while technology vendors often promise seamless and user-friendly systems, the reality on the frontline can be one of significant friction. Reports from implementation studies highlight that a lack of interoperability between different hardware and software systems, coupled with equipment malfunctions, can cause considerable staff frustration and impede the adoption of the model. This disparity between the promise of integrated technology and the experienced reality of disconnected systems represents a critical implementation hurdle. It underscores the need for procurement processes to prioritise proven interoperability and user-centric design to ensure that the technology empowers, rather than burdens, the clinical workforce.
The Intelligence Layer: AI-Driven Triage and Risk Stratification
While Remote Patient Monitoring provides the data stream for virtual wards, Artificial Intelligence (AI) provides the intelligence layer that transforms this raw data into actionable clinical insights. AI's role is not singular; it performs two distinct but critically linked triage functions. The first is a population-level triage to identify the right patients for admission through predictive risk stratification. The second is a continuous patient-level triage to analyse RPM data from admitted patients, prioritising clinical intervention and managing risk at a distance. This section explores the principles of AI triage and its dual application in the virtual ward pathway.
3.1 Principles of AI-Driven Triage
AI-driven triage is the use of intelligent algorithms to rapidly assess a patient's condition and direct them to the most appropriate care pathway. Unlike traditional, manual triage, which relies on a clinician's subjective assessment at a single point in time, AI systems can analyse vast, multi-modal datasets continuously and consistently.
The core mechanism involves several stages. First is data ingestion, where the system pulls in real-time data from multiple sources, such as Electronic Health Records (EHRs), patient-entered symptoms, and data from medical devices. Next, feature extraction processes this raw data, structuring it into a format that the AI can analyse. The assessment engine, which is the heart of the system, then uses machine learning (ML) models and pre-programmed logic to evaluate the case, calculate a risk level, and determine the urgency. Finally, an alert dispatch module notifies the appropriate clinical team through dashboards or secure messaging.
The primary advantages of AI over human triage lie in its speed, consistency, and scalability. An AI system can assess and categorise patients in seconds, operating 24/7 without fatigue or the cognitive biases that can affect human judgment under pressure. This leads to more standardised initial assessments, improved patient flow, and more efficient allocation of scarce clinical resources.
3.2 From Prediction to Prevention: AI-Powered Risk Stratification
One of the most powerful applications of AI in the context of virtual wards occurs before a patient is even admitted. AI-powered risk stratification acts as the intelligent "front door" to the service, proactively identifying patients in the community who are at high risk of an imminent, unplanned hospital admission.
This process uses predictive modelling on large-scale health datasets, combining information from primary care records, previous hospital admissions, demographic data, and indicators of deprivation. Sophisticated algorithms, such as those used in the Johns Hopkins Adjusted Clinical Group (ACG) system, analyse these factors to generate a risk score for each individual, quantifying their likelihood of an emergency hospitalisation within the next 12 months.
NHS teams can then use these risk scores to target preventative interventions. Patients identified as having the highest risk—for example, the top 0.5% of the population who are nearly 19 times more likely than average to be admitted—are considered for proactive admission to a virtual ward. This allows the MDT to provide a period of intensive, multidisciplinary case management aimed at stabilising their condition, optimising their care plan, and addressing the underlying factors contributing to their risk, thereby preventing the predicted hospital admission from ever occurring. This ensures that the resource-intensive virtual ward service is targeted at patients who are not only at high risk but are also "impactable"—that is, those most likely to benefit from the intervention.
3.3 Enhancing Remote Monitoring with Intelligent Systems
Once a patient is admitted to the virtual ward, AI's second triage function comes into play: enhancing the continuous remote monitoring process. The constant stream of data from RPM devices can be overwhelming for clinical teams to manage manually. AI provides the tools to analyse this data intelligently and prioritise clinical action.
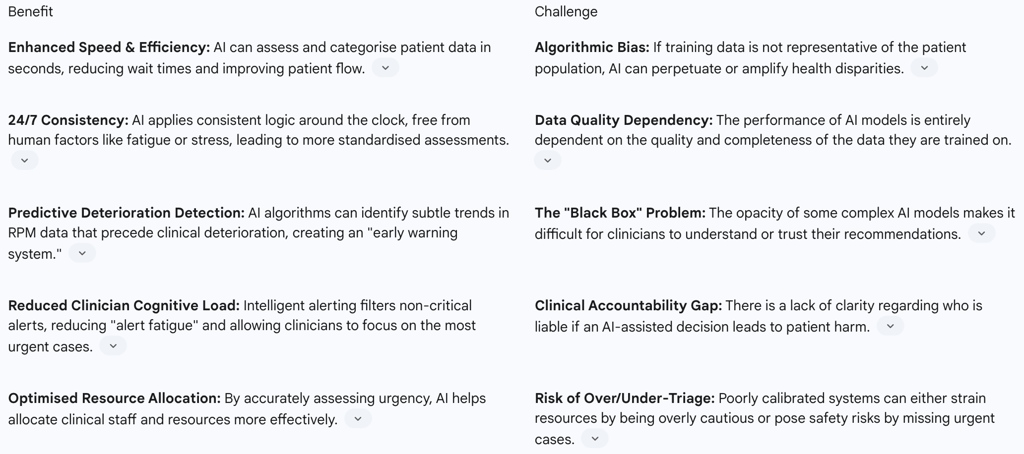

Table 2: AI Triage in RPM - Benefits vs. Challenges
Predictive Analytics for Early Intervention: Rather than simply reacting to a single out-of-range reading, AI algorithms establish a personalised baseline for each patient and analyse trends over time. By processing multimodal data—vital signs, activity levels, patient-reported symptoms—machine learning models can identify complex patterns invisible to the human eye that predict an impending health event, such as a cardiac episode or a COPD exacerbation. This transforms the care model from being reactive to being truly proactive, enabling clinicians to intervene before a crisis occurs.
Intelligent Alerting Systems: A major operational challenge of RPM is the risk of "alert fatigue," where clinicians are inundated with so many notifications that they begin to ignore them. AI-powered triage systems directly address this by filtering the "noise." By learning a patient's individual patterns, the AI can distinguish between a minor, insignificant fluctuation and a meaningful deviation that warrants clinical concern. This intelligent filtering can reduce the number of low-value, non-urgent alerts by over 30%, allowing the clinical team to conserve their cognitive energy for the alerts that matter most.
Workflow Automation: AI also contributes by automating more routine aspects of the virtual ward workflow. This can include sending personalised medication reminders or educational content to patients to improve engagement and adherence. Furthermore, Natural Language Processing (NLP) can be used to analyse and structure symptom descriptions shared by patients via text or voice messages, automatically populating the clinical record and saving valuable clinician time.
These two distinct AI triage functions—for admission and for intervention—are inextricably linked. The effectiveness of the sophisticated intelligent alerting system managing patients on the ward is fundamentally dependent on the accuracy of the risk stratification model that decides who gets admitted to the ward in the first place. This dependency chain is a critical consideration for any health system seeking to implement this model at scale, as a failure in one part of the AI-powered pathway will inevitably undermine the effectiveness of the other.
Impact Analysis: Evidence, Outcomes, and Economic Realities
The strategic push for AI-supported virtual wards is predicated on their potential to deliver a "triple win": improved patient outcomes and experience, enhanced operational efficiency for the NHS, and better value for the taxpayer. However, as with any large-scale transformation, it is essential to critically evaluate the real-world evidence. This section analyses the reported clinical and patient outcomes from NHS implementations, examines the complex and often-debated economic case, and highlights specific case studies that illustrate the model's impact in practice.
4.1 Measuring Success: Clinical and Patient Outcomes
Evidence emerging from early adopters and pilot programmes across the NHS points towards significant positive impacts on both clinical metrics and patient-reported outcomes.
Several NHS Trusts have reported substantial reductions in hospital usage. A virtual ward pilot at North East London NHS Foundation Trust (NELFT), for instance, demonstrated a 45% reduction in admissions and was credited with saving between 400 and 500 hospital bed days over a seven-month period. Similarly, a ground-breaking virtual ward for patients with Chronic Obstructive Pulmonary Disease (COPD) in Hull and East Yorkshire, using the Lenus Health digital platform, has already achieved a 40% reduction in hospital readmissions. Another innovative application involves High Intensity Use (HIU) services, which use data analytics to proactively identify and support individuals who frequently attend A&E. In several parts of the country, these services have successfully reduced A&E attendances for this cohort by more than half, with one Trust reporting a 58% reduction in attendances and a 62% reduction in admissions.
These system-level benefits are mirrored by consistently high levels of patient satisfaction. The same NELFT pilot that reduced admissions also recorded a 97% patient satisfaction rate. Patients frequently report feeling more empowered and in control of their condition, less anxious, and value the reassurance of continuous monitoring while recovering in the comfort and familiarity of their own home.
These localised findings are supported by broader evidence from systematic reviews. One meta-analysis published in JAMA Network Open found that certain virtual ward models for heart failure patients led to a statistically significant 14% reduction in mortality and a 16% decrease in hospital readmissions compared to standard care.
However, it is crucial to acknowledge a significant "evidence gap." While the case studies are promising, they are often localised, condition-specific, and methodologically diverse. Authoritative bodies like The Health Foundation have cautioned that there is not yet a robust, national-level evidence base to definitively prove that virtual wards reduce overall pressure on hospital beds across the entire system. A key ambiguity remains: it is often unknown how many patients cared for on a virtual ward would have definitively occupied a hospital bed in its absence. This highlights a situation where national policy and investment are, to some extent, running ahead of comprehensive, generalisable evidence, underscoring the urgent need for standardised data collection and rigorous national evaluation.
4.2 The Cost-Effectiveness Conundrum
The economic case for virtual wards is complex and has been the subject of considerable debate. The initial national investment of £450 million over two years necessitates a clear return on investment, but measuring this has proven challenging.
A study from Wrightington, Wigan and Leigh NHS Foundation Trust garnered significant attention when it concluded that the cost of freeing one hospital bed by using a virtual ward was approximately twice that of an inpatient bed day. This headline finding fuelled scepticism about the model's financial viability. However, this perspective is contested by a growing body of evidence pointing to substantial savings. An evaluation of virtual wards across the South East region, for example, reported net savings of over £10 million through reduced hospital usage. Medway NHS Foundation Trust calculated its daily virtual ward cost per patient at just £187, compared to an estimated £657 for an equivalent inpatient. An earlier evaluation of the original Croydon model estimated a cost saving of £742 per patient compared to a control group.
This disparity arises from several factors. Firstly, as previously noted, comparing the cost of a "virtual bed" (a unit of dedicated workforce capacity) with that of a "physical bed" (a share of fixed hospital overheads) is not a like-for-like comparison. Secondly, there is no national consensus on how to measure virtual ward costs and capacity, leading to inconsistent and often incomparable local analyses. Finally, a narrow focus on per-diem costs fails to capture the broader, and often non-monetised, value of the virtual ward model. A more holistic economic assessment would need to account for the value of improved patient experience, the avoidance of hospital-acquired harms (which carry their own significant costs), and the strategic benefit of having a flexible, scalable capacity that can be expanded during periods of peak demand. The current debate is often overly simplistic, and a more nuanced approach to value assessment is required. To address these shortcomings, NHS England is developing a national minimum dataset for virtual wards, which aims to standardise data collection and enable more robust economic analysis, though this is not expected to be fully implemented until 2026.
4.3 Case Studies in Focus
Examining specific implementations provides concrete insights into how AI-supported virtual wards are functioning in the NHS.
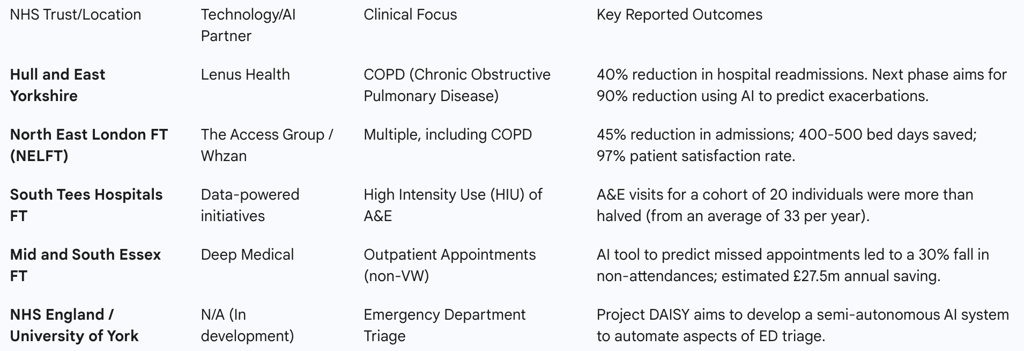
Table 3: Summary of NHS Trust AI/VW Case Study Outcomes
Hull and East Yorkshire (Lenus Health): This case demonstrates the evolution of a virtual ward. The initial phase used a digital platform to empower COPD patients with self-monitoring tools, leading to a 40% drop in readmissions. The crucial next step involves leveraging the data collected through this platform. AI will be used to analyse patient-reported symptoms and physiological data to identify trends and triggers that predict an impending exacerbation, allowing the clinical team to intervene proactively before the patient becomes acutely unwell. This represents a shift from enhanced monitoring to true predictive care.
Mid and South Essex NHS Foundation Trust (Deep Medical): While not a virtual ward for acute care, this pilot is highly relevant as it showcases the power of predictive AI in managing patient flow and system capacity. The AI tool predicts the likelihood of a patient missing an outpatient appointment by analysing a range of data, including external factors like weather and traffic. This allows the Trust to intelligently overbook clinics or offer slots to alternative patients, slashing non-attendance rates by 30%. The principles of using predictive analytics to optimise resource allocation are directly transferable to managing admissions and discharges for virtual wards.
Project DAISY (University of York): This developmental project represents a future-facing vision for AI triage at the very front door of the hospital. DAISY (Diagnostic AI System for Robot-Assisted ED Triage) is a semi-autonomous system designed to guide patients through an initial assessment, capturing both subjective symptoms and objective vital signs. The AI algorithm then provides a preliminary diagnosis and a suggested investigation plan for review by a human clinician. This aims to streamline the entire ED triage process, reduce waiting times, and quickly identify the most critically ill patients. It exemplifies the ambition to embed AI more deeply into core clinical decision-making processes.
Navigating the Implementation Maze: Challenges and Stakeholder Perspectives
Despite the strategic imperative and promising early results, the widespread and effective implementation of AI-supported virtual wards is fraught with significant challenges. These hurdles are not primarily technological in nature; rather, they are deeply embedded in the human, systemic, and ethical dimensions of healthcare transformation. Successfully scaling this model requires a clear-eyed understanding of the perspectives of clinicians and patients, the systemic barriers within the NHS, and the complex regulatory landscape.
5.1 The Human Factor: Clinician and Patient Perspectives
The success of any new care model hinges on its acceptance by those who deliver and receive the care.
Clinician Trust and Workload: There is a palpable tension in clinicians' views on AI. While many recognise its potential to streamline workflows and triage radiology scans, for example , there is also a deep-seated scepticism born from past experiences with "digitalisation's empty promises" that increased, rather than decreased, administrative burdens. A core concern is that AI, particularly in complex triage scenarios, cannot replicate the nuanced clinical judgment that accounts for a patient's full medical and social history. For AI to be adopted, clinicians must trust it as a supportive tool that augments their expertise, not as a replacement for it. This trust is fragile and heavily dependent on the technology's reliability and its ability to integrate seamlessly into established clinical workflows. Implementations that fail to engage in co-design with frontline staff risk creating solutions that are seen as a hindrance rather than a help.
Patient Acceptance and Digital Equity: From the patient's perspective, the virtual ward model offers the welcome prospect of recovering at home. However, their acceptance of the AI-driven technologies that enable it is conditional. Patients express significant concerns around data privacy and security, the accuracy of AI-generated advice, and a strong desire for human oversight in all critical decisions. Furthermore, the model's reliance on digital technology raises profound questions of equity. Patient suitability for a virtual ward is often determined not just by their clinical condition, but by their digital literacy, access to reliable internet connectivity, and the level of social and familial support they have at home. This creates a significant risk of widening health inequalities, where the benefits of this innovative care model are only accessible to more affluent, tech-savvy, and well-supported segments of the population, while excluding many of the vulnerable, elderly, and isolated individuals who could benefit most. This issue of digital equity is not an ancillary concern but a core challenge to the model's ethical legitimacy and its ability to deliver on the NHS's founding principle of universal access.
Cultural Shift: The transition from in-person to remote acute care represents a major cultural shift for the entire healthcare system. It introduces ambiguity and uncertainty around established workflows, professional responsibilities, and the very identity of the service.
5.2 Systemic Hurdles: Technology, Data, and Workforce
Beyond individual perspectives, there are formidable systemic barriers to implementation at scale.
Interoperability and Infrastructure: A persistent and critical challenge is the lack of interoperability between the myriad of digital systems used across the NHS. Virtual ward platforms must be able to exchange data seamlessly with hospital EHRs, primary care systems, and community service records. The reality is often one of data silos, requiring manual data re-entry and causing significant staff frustration. The foundational digital infrastructure of the NHS is not yet consistently fit for the purpose of supporting these advanced, data-intensive models of care.
Data Quality and Governance: The adage "garbage in, garbage out" is acutely true for AI in healthcare. The performance of risk stratification and triage algorithms is entirely dependent on access to high-quality, comprehensive, and unbiased data. Ensuring the integrity of this data and establishing robust governance for its use is a fundamental prerequisite for the safe deployment of AI.
Workforce and Training: Virtual wards demand a new set of skills from the clinical workforce. Staff require training not only in using the specific technologies but also in new clinical pathways for managing risk at a distance, interpreting trend data, and conducting effective virtual consultations. There is a need to develop new career paths and competencies for a "virtualist" workforce, a challenge compounded by existing workforce shortages across the NHS.
5.3 The Ethical and Regulatory Framework
The use of AI in high-stakes clinical decision-making introduces a new layer of ethical and regulatory complexity.
Accountability and Liability: Perhaps the most significant unresolved issue is that of accountability. If an AI triage system makes an incorrect assessment that leads to patient harm, who is responsible? Is it the clinician who accepted the AI's recommendation, the NHS Trust that procured the system, or the company that developed the algorithm?. This ambiguity creates a major barrier to adoption. Clinicians feel ultimately responsible for their patients, yet they are being asked to trust and act upon the outputs of "black box" algorithms whose internal logic they cannot fully inspect or understand. This fundamental tension between the opacity of some AI models and the principle of clinical accountability must be resolved through clear national guidance and regulatory frameworks to build the necessary confidence for widespread use. The most successful AI tools will likely be those that prioritise "explainability" (XAI), providing visualisations or rationales for their recommendations to bridge this trust gap.
Algorithmic Bias: There is a substantial risk that AI systems, if trained on historical datasets that reflect existing societal biases, will perpetuate or even amplify health inequalities. An algorithm trained predominantly on data from one demographic group may be less accurate when applied to another, leading to poorer outcomes for underrepresented populations. Recognizing this risk, the NHS has begun piloting the use of Algorithmic Impact Assessments (AIAs), a process designed to compel developers to assess and mitigate potential biases before their systems are deployed on NHS data.
Data Privacy and Security: The operation of AI-supported virtual wards involves the collection and processing of large volumes of highly sensitive personal health data. This necessitates strict adherence to data protection laws, including the UK GDPR and the common law duty of confidentiality. A comprehensive Data Protection Impact Assessment (DPIA) is a legal requirement before any such system is implemented, to identify and mitigate risks to patient privacy. Robust technical and organisational security measures, including encryption, access controls, and legally binding data processing agreements with technology vendors, are essential to safeguard patient confidentiality and maintain public trust.
Strategic Outlook and Recommendations
The convergence of virtual wards, remote patient monitoring, and artificial intelligence is not a transient trend but a foundational element of the long-term strategic direction for the NHS. This final section situates the AI-supported virtual ward model within the broader context of national healthcare policy, provides actionable recommendations for key stakeholders to navigate the challenges identified in this report, and offers a vision for the future evolution of at-home acute care.
6.1 Aligning with National Strategy
The virtual ward initiative is deeply interwoven with the UK government's and NHS England's flagship strategies for healthcare reform. It is a direct and practical manifestation of the core principles outlined in the NHS Long Term Plan and the subsequent 10-Year Health Plan for England.
These strategies articulate a clear vision for shifting the NHS from a 20th-century "analogue" service to a 21st-century "digital" one. A central tenet of this transformation is moving the locus of care away from the traditional hospital setting and closer to the patient. The plan explicitly states that care should be delivered "digitally by default," "in a patient's home if possible," and only "in a hospital if necessary". AI-enabled virtual wards are the primary vehicle for achieving this ambition for the acute care sector.
Furthermore, the national strategy identifies AI as a key technology to be harnessed, with the ambitious goal of making the NHS a world leader in its application. The plan envisions AI being seamlessly integrated into clinical pathways to speed up diagnosis, revolutionise outpatient services, and free up clinician time. Remote monitoring, supported by a significant national scaling programme, is seen as the mechanism to make proactive management of patients the new normal. To facilitate this, national procurement frameworks, such as the "Technology Enabled Care Services 2" agreement, have been established to streamline the process for Integrated Care Systems (ICSs) to acquire the necessary virtual ward technologies and build regional capacity.
6.2 The Path Forward: Strategic Recommendations
To successfully navigate the implementation maze and realise the full potential of this strategic vision, a concerted and coordinated effort is required from all stakeholders. Based on the analysis conducted in this report, the following recommendations are proposed:
For Policymakers (Department of Health and Social Care, NHS England)
Prioritise Foundational Infrastructure over Standalone Technologies: The current focus on procuring individual AI tools and platforms should be balanced with a greater strategic investment in the foundational digital infrastructure of the NHS. This includes accelerating the rollout of fully interoperable Electronic Health Records, establishing and enforcing national data standards, and creating secure data-sharing environments. A robust digital foundation is the most critical enabler for any AI tool to function effectively and safely at scale.
Develop a National Framework for AI Accountability: The ambiguity surrounding clinical liability for AI-assisted decisions is a major impediment to clinician trust and adoption. A clear national framework must be developed, in collaboration with medical royal colleges, regulators, and indemnity providers, to define the lines of responsibility between clinicians, healthcare organisations, and technology developers. This will provide the legal and professional certainty needed for safe innovation.
Mandate and Fund Digital Equity Initiatives: Digital inclusion must be treated as a core safety and quality requirement, not an optional extra. National funding for virtual wards should be explicitly tied to the implementation of digital equity plans. This must include resources for providing patients with the necessary technology (e.g., pre-configured tablets with data plans), offering training and support for patients and their carers, and developing non-digital pathways for those who cannot or will not use the technology. This is essential to prevent the creation of a two-tier system of access to acute care.
For NHS Leaders (Trust and ICS Executives)
Adopt a "Co-design" Implementation Model: Technology procurement and service design must not be a top-down process. From the outset, frontline clinicians, administrative staff, and diverse patient and carer groups must be involved in defining the problem, selecting the technology, and redesigning the clinical pathway. This co-design approach is critical for ensuring that solutions are clinically appropriate, integrate with existing workflows, and are trusted by both staff and patients.
Invest in Workforce Transformation: The shift to virtual wards requires more than just new technology; it requires a new type of workforce. A strategic plan for workforce development is needed, including the creation of new roles (e.g., "virtualist" clinicians, data analysts), the development of training programs focused on remote risk management and virtual consultation skills, and the establishment of clear career pathways within this emerging field of practice.
Demand Explainable AI (XAI) in Procurement: When procuring AI-driven triage and monitoring systems, executives should make transparency and explainability a key selection criterion. Prioritise solutions that can provide a clear rationale for their recommendations, even if this involves a marginal trade-off in predictive accuracy. This is essential for bridging the trust gap with clinicians and ensuring they can maintain their professional accountability.
For Technology Developers
Engineer for Seamless Integration: Interoperability should be a primary design principle, not an afterthought. Solutions should be built with open APIs and a demonstrable commitment to integrating seamlessly with the major EHR and clinical information systems used across the NHS. This will significantly reduce the friction of adoption and the burden on frontline staff.
Validate Algorithms on Diverse UK Datasets: To combat the significant risk of algorithmic bias, developers must proactively train and validate their models on datasets that are genuinely representative of the UK's diverse population. Performance metrics should be transparently reported across different demographic groups (e.g., by ethnicity, age, and socioeconomic status) to allow for proper scrutiny by procurers and regulators.
6.3 The Future Vision: The Evolution of At-Home Acute Care
The AI-supported virtual ward model being implemented in the NHS is part of a global movement towards decentralised, technology-enabled healthcare. International examples offer a glimpse of the future trajectory. In China, the "Smart Home Ward" concept is exploring a deeper integration of healthcare into the patient's living environment, using 5G, the Internet of Things (IoT), and ambient sensors to create a truly intelligent home care ecosystem. Meanwhile, leading hospitals in Europe are demonstrating the power of highly integrated clinical software to optimise workflows and patient flow across entire health systems.
In the coming years, the technology underpinning virtual wards will continue to evolve. We can expect to see the integration of more sophisticated passive monitoring through wearables and ambient sensors, reducing the burden of manual data entry for patients. Generative AI will likely play a greater role in automating clinical documentation and facilitating more natural, conversational interactions between patients and care systems.
Ultimately, the strategic goal extends beyond simply moving the hospital bed into the home. The continuous data streams and predictive analytics capabilities of these systems are a critical stepping stone towards a fundamental paradigm shift in healthcare: from a system that reacts to illness to one that proactively predicts risk and prevents disease. The AI-supported virtual ward is a vital learning ground for the skills, technologies, and clinical pathways that will define the future of a more sustainable, proactive, and personalised health service.
